
News
Lithium battery overcharge mechanism and overcharge prevention measures
Labor Frontier of lithium battery 2022-05-27 22:56 Published in Guangdong
Overcharging is one of the most difficult items to pass in the current lithium battery safety test, so it is necessary to understand the overcharging mechanism and the current measures to prevent overcharging.
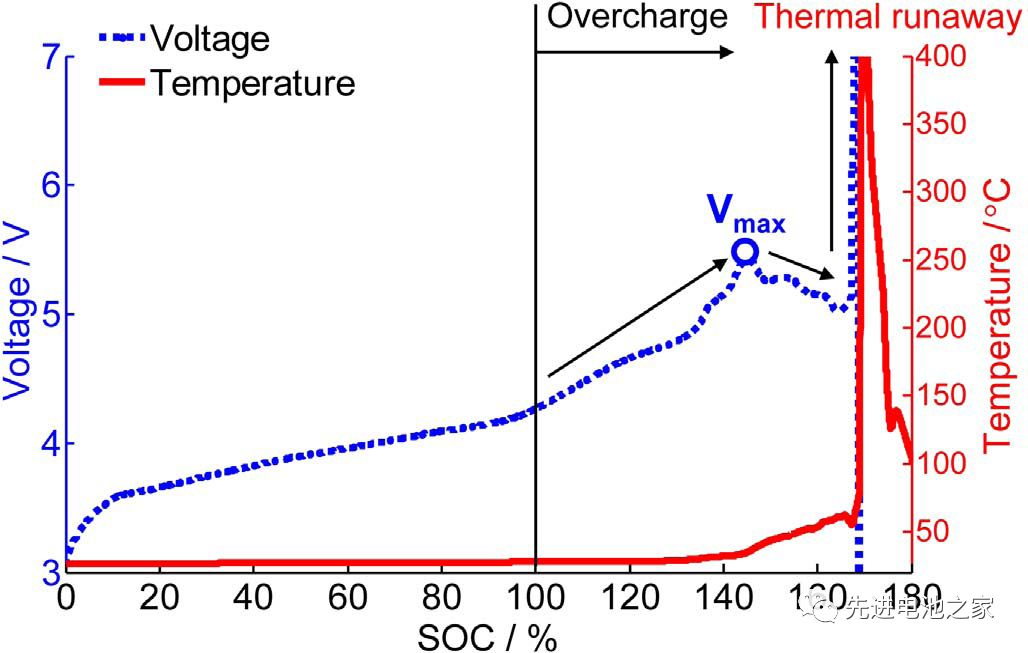
Figure 1 shows the voltage and temperature curves of the NCM+LMO/Gr system when the battery is overcharged. The voltage reaches a maximum at 5.4V, and then the voltage drops, eventually causing thermal runaway. The voltage and temperature curves of ternary battery overcharge are very similar.
Overcharging is one of the most difficult items to pass in the current lithium battery safety test, so it is necessary to understand the overcharging mechanism and the current measures to prevent overcharging.
Figure 1 shows the voltage and temperature curves of the NCM+LMO/Gr system when the battery is overcharged. The voltage reaches a maximum at 5.4V, and then the voltage drops, eventually causing thermal runaway. The voltage and temperature curves of ternary battery overcharges are very similar.
Picture 1.png
When the lithium battery is overcharged, heat and gas will be generated, and the heat includes ohmic heat and heat generated by side reactions, of which ohmic heat is dominant. The side reactions of the battery caused by overcharge are firstly the insertion of excess lithium into the negative electrode, and lithium dendrites will grow on the surface of the negative electrode (the N/P ratio will affect the initial SOC of the growth of lithium dendrites). The second is that excess lithium is extracted from the positive electrode, causing the positive electrode structure to collapse, releasing heat and releasing oxygen. Oxygen will accelerate the decomposition of the electrolyte, the internal pressure of the battery will continue to rise, and the safety valve will open after a certain level. The contact of the active substance with the air further generates more heat.
Studies have shown that reducing the amount of electrolyte can significantly reduce heat and gas generation during overcharging. In another study, when the battery is overcharged without a splint or the safety valve cannot be opened normally, the battery is prone to explosion.
A slight overcharge will not cause thermal runaway, but will cause capacity fade. The study found that when the battery with NCM/LMO hybrid material as the positive electrode is overcharged, the capacity of SOC is lower than 120% without obvious attenuation, and when the SOC is higher than 130%, the capacity will be attenuated significantly.
At present, there are several methods to solve the overcharge problem:
1) The protection voltage is set in the BMS, usually the protection voltage is lower than the peak voltage during overcharge;
2) Improve the overcharge resistance of the battery through material modification (such as material coating);
3) Add anti-overcharge additives to the electrolyte, such as redox pairs;
4) The use of voltage-sensitive membrane, when the battery is overcharged, the membrane resistance is significantly reduced, which plays a role in shunting;
5) The use of OSD and CID design in the square aluminum shell battery is currently a general anti-overcharge design. A similar design cannot be achieved with pouch cells.
references
Energy Storage Materials 10 (2018) 246–267
This time, we will introduce the voltage and temperature changes of lithium cobalt oxide batteries during overcharge. The figure below is the overcharge voltage and temperature curve of the lithium cobalt oxide battery, and the horizontal axis is the delithiation amount. The negative electrode is graphite, and the electrolyte solvent is EC/DMC. The battery capacity is 1.5Ah. The charging current is 1.5A, and the temperature is the internal temperature of the battery.
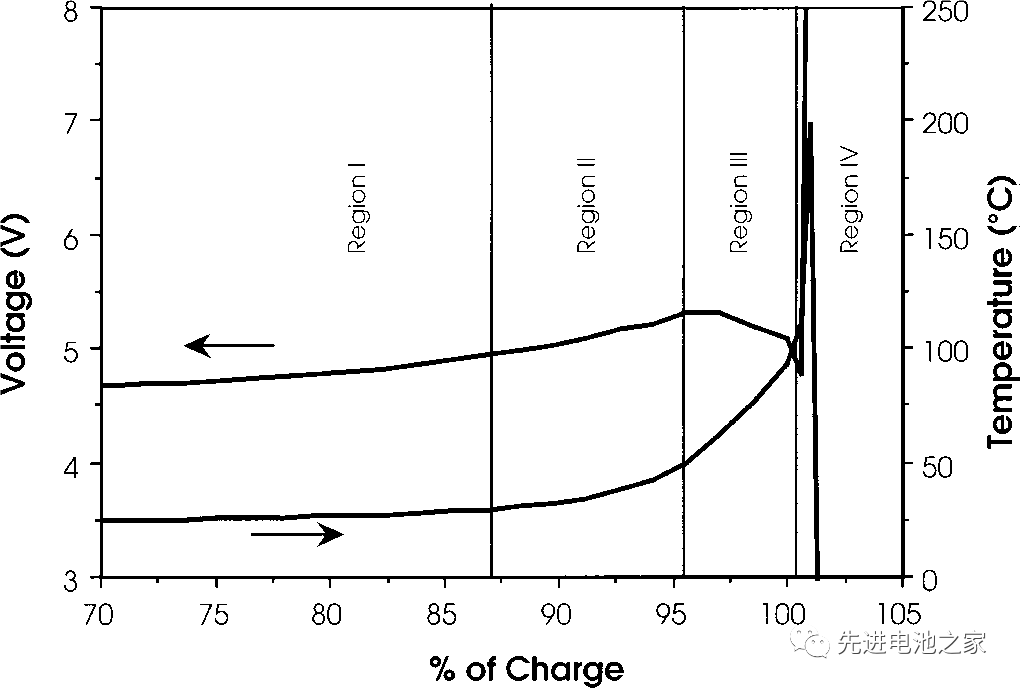
Lithium battery overcharge can be roughly divided into 4 areas, and the characteristics of each area are as follows:
Zone I
1. The battery voltage rises slowly. The lithium cobalt oxide positive electrode delithium exceeds 60%, and metal lithium is precipitated on the negative electrode side.
2. The battery is bulging, possibly due to high pressure oxidation of the electrolyte on the positive side.
3. The temperature is basically stable, with a slight rise.
Zone II
1. The temperature begins to rise slowly.
2. In the range of 80~95%, the positive resistance increases and the internal resistance of the battery increases, but it decreases at 95%.
3. The battery voltage exceeds 5V and reaches the highest.
Zone III 1. Around 95%, the battery temperature begins to rise rapidly. 2. Starting at about 95%, the battery voltage drops slightly until approaching 100%. 3. When the internal temperature of the battery reaches about 100°C, the battery voltage drops sharply, which may be caused by the decrease in the internal resistance of the battery caused by the increase in temperature. Zone IV 1. When the internal temperature of the battery is higher than 135°C, the PE diaphragm begins to melt, the internal resistance of the battery rises rapidly, the voltage reaches the upper limit (~12V), and the current drops to a lower value. 2. Between 10-12V, the battery voltage is unstable and the current fluctuates.
3. The internal temperature of the battery rises rapidly, and the temperature rises to 190-220°C before the battery ruptures.
4. The battery is ruptured.
The ternary battery overcharge is similar to the lithium cobalt oxide battery. When the ternary square aluminum shell battery on the market is overcharged, it is roughly controlled that the OSD or CID starts when entering the III zone, and the current is cut off to protect the battery from overcharging.
References
Journal of The Electrochemical Society,148 (8) A838-A844 (2001)
Lithium battery overcharge mechanism and overcharge prevention measures (3)
In this paper, the overcharge performance of a 40Ah pouch battery with a positive electrode of NCM111+LMO is studied through experiments and simulations. The overcharge currents are 0.33C, 0.5C and 1C, respectively. The battery size is 240mm * 150mm * 14mm. (Calculated according to the rated voltage of 3.65V, its volume specific energy is about 290Wh/L, and the specific energy is still relatively low)
The voltage, temperature and internal resistance changes during overcharge are shown in Figure 1. It can be roughly divided into four stages:
The first stage: 1<SOC<1.2, there is no obvious side reaction inside the battery, and the battery temperature and internal resistance change little.
The second stage: 1.2<SOC<1.4, Mn in the positive electrode dissolves, the electrolyte on the positive electrode side is oxidized, and metal lithium is precipitated on the negative electrode surface. The reaction of metallic lithium with the solvent makes the SEI film thicker, the battery impedance increases, and the battery temperature begins to rise slowly.
The third stage: 1.4 < SOC < 1.6, the battery temperature rises faster, the battery swells obviously, the oxidation of the electrolyte on the positive side accelerates, and a large amount of heat and gas is released. The metal lithium continues to precipitate on the surface of the negative electrode, the SEI film begins to decompose, and the lithiated graphite reacts with the electrolyte. Due to the change in the structure of the cathode material, the cell voltage decreased slightly after reaching a peak value of 5.2 V.
The fourth stage: SOC>1.6, the internal pressure of the battery exceeds the limit, the casing ruptures, the diaphragm shrinks and deforms, and the battery thermally runs out of control. A short circuit occurs inside the battery, a large amount of energy is rapidly released, and the battery temperature rises sharply to 780°C
。
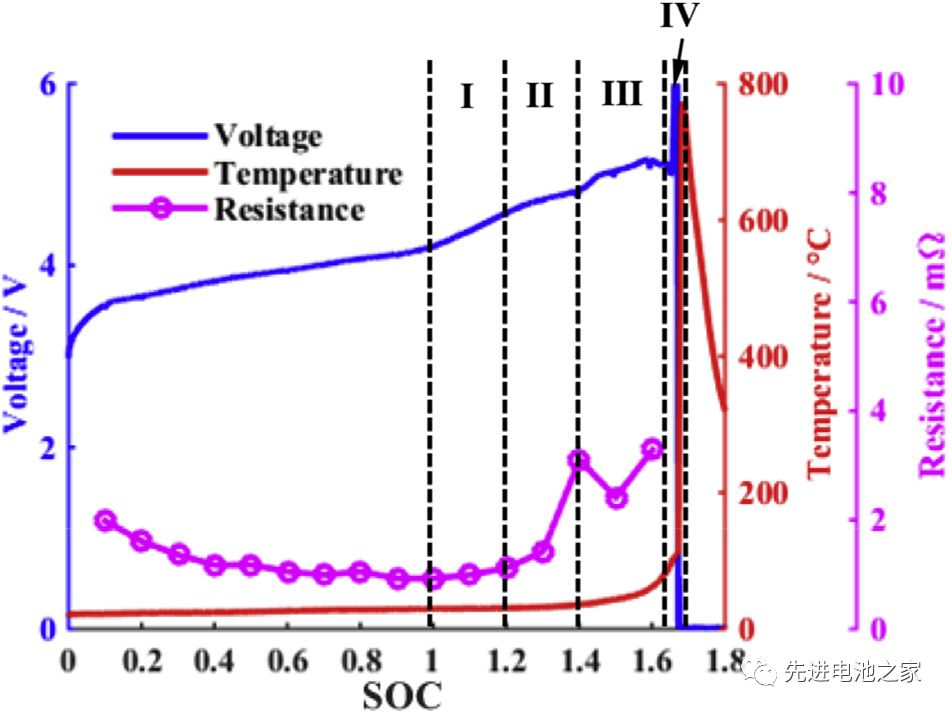
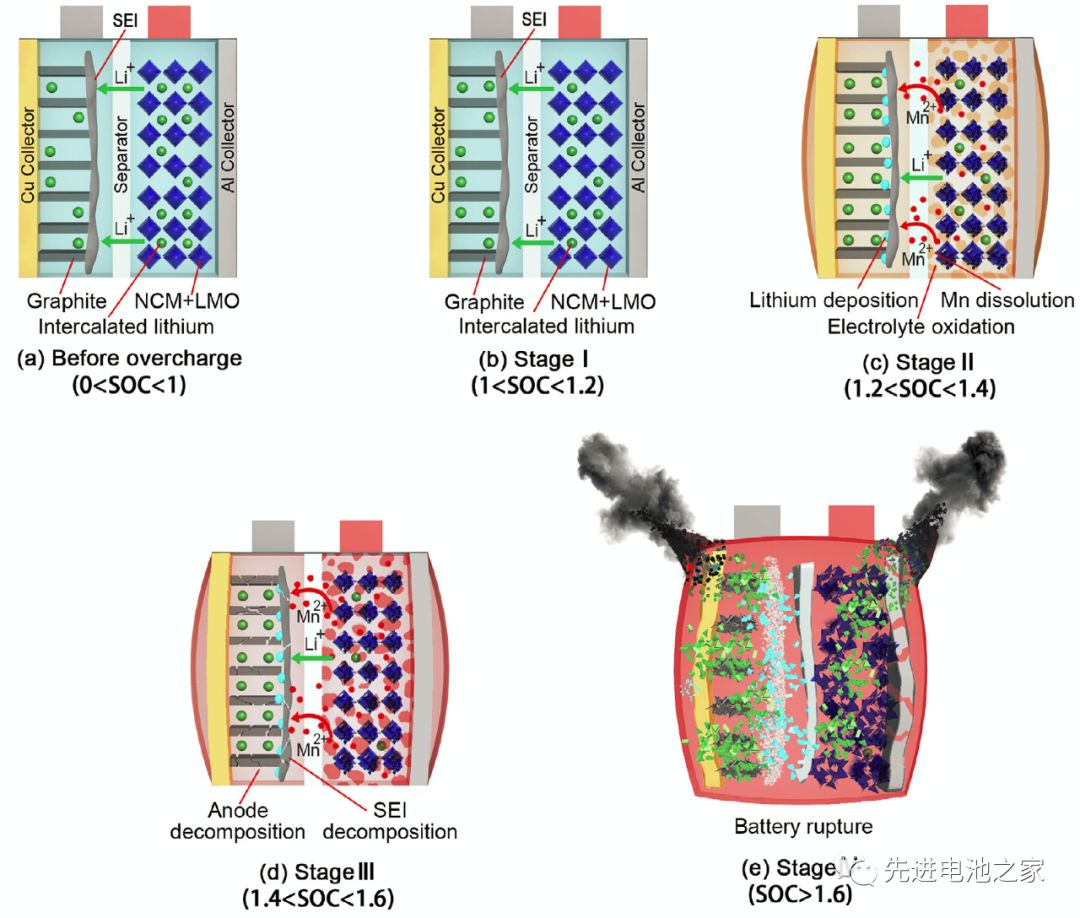
Figure 2
The heat generation during the overcharging process includes: reversible entropy heat, Joule heat, chemical reaction heat and heat released by internal short circuit. The heat of chemical reaction includes the heat released from the dissolution of Mn, the reaction of lithium metal with the electrolyte, the oxidation of the electrolyte, the decomposition of the SEI film, the decomposition of the negative electrode, and the decomposition of the positive electrode (NCM111 and LMO). Table 1 shows the enthalpy change and activation energy of each reaction. (This article ignores the side reaction of the binder)
Table 1
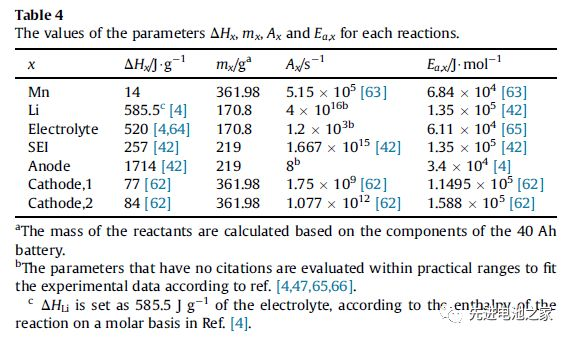
Figure 3 is a comparison of the heat generation rate when overcharging with different charging currents. From Figure 3 the following conclusions can be drawn:
1) As the charging current increases, the thermal runaway time advances.
2) The heat generation in overcharging is mainly Joule heat. SOC<1.2, the total heat production is basically equal to Joule heat.
3) In the second stage (1<SOC<1.2), three types of side reactions, such as Mn dissolution, metal lithium and electrolyte reaction, and electrolyte oxidation, start to react successively. When the current is 1C, the reaction will advance.
4) When SOC>1.45, the heat released by the reaction between metal lithium and the electrolyte will exceed the Joule heat.
5) When the SOC>1.6, the decomposition reaction of the SEI film and the anode begins, the heat production rate of the electrolyte oxidation reaction increases sharply, and the total heat production rate reaches the peak value. (The descriptions of 4 and 5 in the literature are somewhat inconsistent with the figures. Here, the figures shall prevail and have been adjusted.)
6) During the overcharge process, the reaction between metal lithium and the electrolyte and the oxidation of the electrolyte are the main reactions.
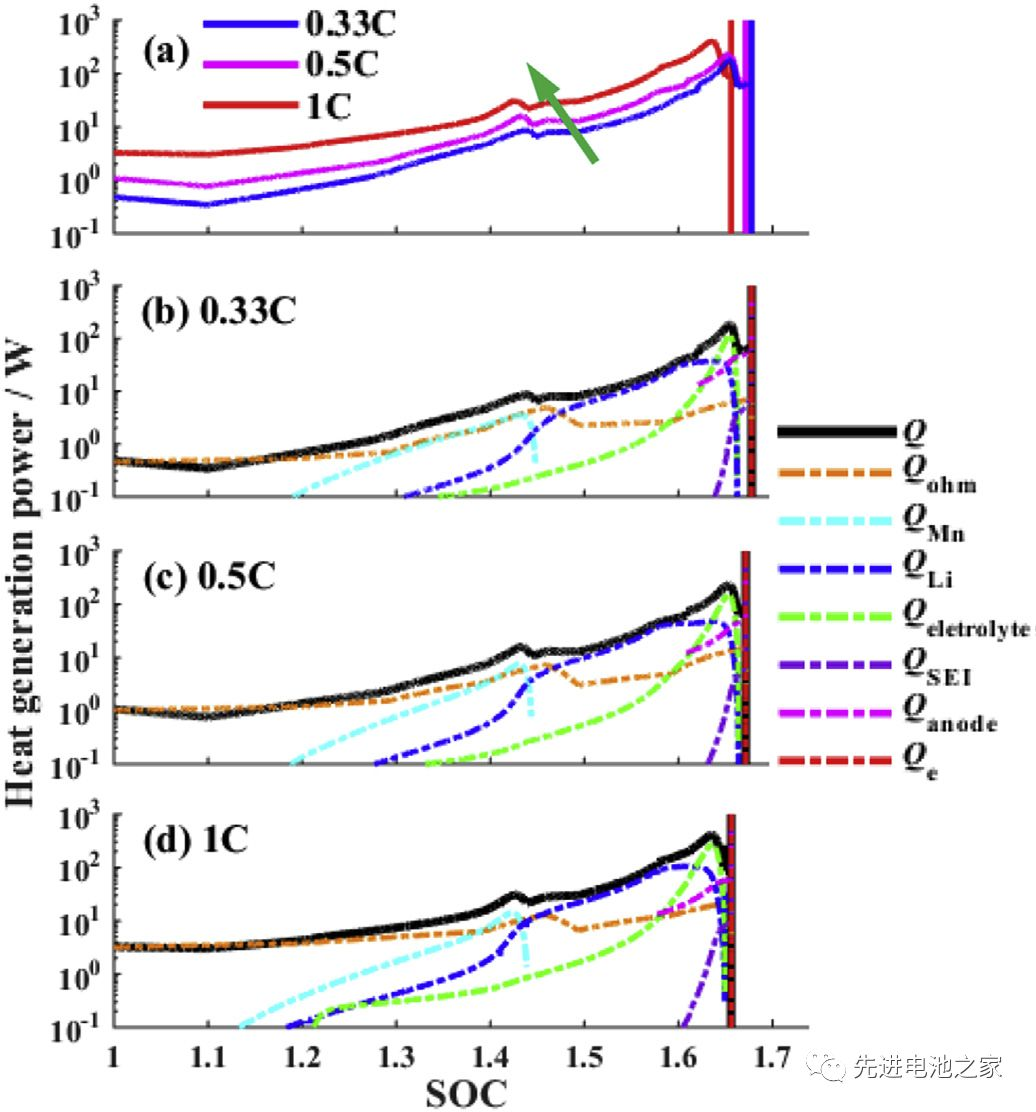
Through the above analysis, electrolyte oxidation potential, anode capacity and thermal runaway onset temperature are the three key parameters of overcharge. Figure 4 shows the effect of three key parameters on overcharge performance. It can be seen that the improvement of the oxidation potential of the electrolyte can greatly improve the overcharge performance of the battery, while the negative electrode capacity has little effect on the overcharge performance. (In other words, the high-voltage electrolyte helps to improve the overcharge performance of the battery, and increasing the N/P ratio has little effect on the overcharge performance of the battery.)
Those who are interested can read the literature further.
References
D. Ren et al. Journal of Power Sources 364(2017) 328-340

